Abstract
Background
The logistical and financial challenges posed by the current multi-dose Human Papillomavirus (HPV) vaccines in countries with limited resources, result in low vaccine coverage and thus are big hurdles against effective prevention for cervical cancer. This study compared the efficacy of a solitary dose of HPV vaccine regimen with standard multi-doses.
Methods
The PRISMA guidelines were followed. A thorough search was conducted till January 10, 2024, to identify studies investigating the HPV vaccine's efficacy with single versus multiple doses or no dose. For the meta-analysis, a random-effects model was used, considering heterogeneity with I² statistics. The included studies were also assessed for quality using standard tools.
Results
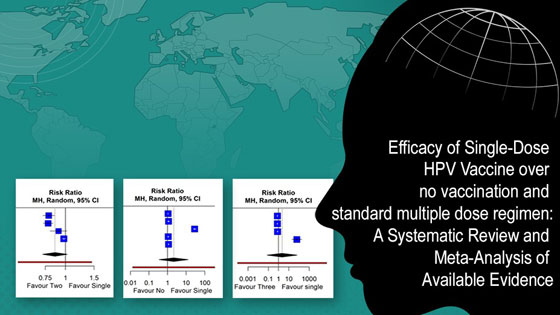
Three observational studies were included with two randomized controlled trials. The meta-analysis comparing individuals given a single dose of the HPV vaccine to those with no dose, revealed an insignificant RR (risk ratio) of 2.11 (95%CI [0.34; 12.87]) with high heterogeneity, which on adjusting for outlier, became significantly better (RR of 1.09 (95%CI [1.04; 1.14]); I² = 33.6%). The comparison of single-dose with two-dose regimens showed no significant difference, while that with three-dose regimens revealed an RR of 2.21 (95%CI [0.07; 66.28]), with I² = 95%. However, a leave-one-out analysis with two- and three-doses, indicated significantly less protection with single dose, RR of 0.81 (95%CI [0.67;0.98]) and 0.78 (95% CI [0.77; 0.79]), respectively. The included studies and trials had moderate to high quality.
Conclusion
A single-dose HPV vaccine regimen could offer a potential interim solution in preventing incident HPV infection, especially in resource-limited settings (like LMICs) where simplifying vaccination logistics and reducing costs could significantly enhance vaccine accessibility and coverage. However, more evidence is needed to confirm these results and assess the long-term effectiveness of the single-dose regimen.
Keywords:
hpv, vaccine efficacy, cervical cancer, single dose, multiple doses, Systematic Review, Meta-analysisReferences
Beaulieu N, Bloom D, Bloom R, Stein R. Breakaway: The global burden of cancer—challenges and opportunities. Economist Intell Unit. 2009.
Jani C, Abdallah N, Mouchati C, Jani R, Sharma R, Bhatt P, et al. Trends of kidney cancer burden from 1990 to 2019 in European Union 15+ countries and WHO regions. Sci Rep. 2022;12(1):22368.
Basu P, Malvi SG, Joshi S, Bhatla N, Muwonge R, Lucas E, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol. 2021;22(11):1518-29.
Eala MAB, Tantengco OAG. Global online interest in cervical cancer care in the time of COVID-19: An infodemiology study. Gynecol Oncol Rep. 2022;41:100998.
Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):575-90.
Amboree TL, Wermuth PP, Montealegre JR, Fujimoto K, Mgbere O, Darkoh C. Sexual Behaviors and Human Papillomavirus Vaccination in a Heterosexually Active Adult Population at Increased Risk for HIV Infection. Arch Sex Behav. 2023;52(2):793-801.
Osaghae I, Darkoh C, Chido-Amajuoyi OG, Chan W, Wermuth PP, Pande M, et al. Association of provider HPV vaccination training with provider assessment of HPV vaccination status and recommendation of HPV vaccination. Hum Vaccin Immunother. 2022;18(6):2132755.
Hsiao A, Struckmann V, Stephani V, Mmbando D, Changalucha J, Baisley K, et al. Costs of delivering human papillomavirus vaccination using a one-or two-dose strategy in Tanzania. Vaccine. 2023;41(2):372-9.
Borghi J, Chalabi Z. Square peg in a round hole: re-thinking our approach to evaluating health system strengthening in low-income and middle-income countries. BMJ Spec J. 2017. p. e000406.
Bulthuis SE, Kok MC, Raven J, Dieleman MA. Factors influencing the scale-up of public health interventions in low-and middle-income countries: a qualitative systematic literature review. Health Policy Plan. 2020;35(2):219-34.
Rabbani F, Shipton L, White F, Nuwayhid I, London L, Ghaffar A, et al. Schools of public health in low and middle-income countries: an imperative investment for improving the health of populations? BMC Public Health. 2016;16(1):1-12.
Herlihy N, Hutubessy R, Jit M. Current Global Pricing For Human Papillomavirus Vaccines Brings The Greatest Economic Benefits To Rich Countries. Health Aff (Millwood). 2016;35(2):227-34.
Hussain R, Bukhari NI, Ur Rehman A, Hassali MA, Babar ZU. Vaccine Prices: A Systematic Review of Literature. Vaccines (Basel). 2020;8(4).
Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med. 2021;144:106399.
Lane S, MacDonald NE, Marti M, Dumolard L. Vaccine hesitancy around the globe: Analysis of three years of WHO/UNICEF Joint Reporting Form data-2015-2017. Vaccine. 2018;36(26):3861-7.
Orumaa M, Kjaer SK, Dehlendorff C, Munk C, Olsen AO, Hansen BT, et al. The impact of HPV multi-cohort vaccination: Real-world evidence of faster control of HPV-related morbidity. Vaccine. 2020;38(6):1345-51.
Sabeena S, Bhat PV, Kamath V, Arunkumar G. Global human papilloma virus vaccine implementation: An update. J Obstet Gynaecol Res. 2018;44(6):989-97.
Joshi S, Anantharaman D, Muwonge R, Bhatla N, Panicker G, Butt J, et al. Evaluation of immune response to single dose of quadrivalent HPV vaccine at 10-year post-vaccination. Vaccine. 2023;41(1):236-45.
Rodriguez AM, Zeybek B, Vaughn M, Westra J, Kaul S, Montealegre JR, et al. Comparison of the long‐term impact and clinical outcomes of fewer doses and standard doses of human papillomavirus vaccine in the United States: a database study. Cancer. 2020;126(8):1656-67.
Zeng Y, Moscicki A-B, Woo H, Hsu C-H, Kemp TJ, Pinto LA, et al. HPV16/18 Antibody Responses After a Single Dose of Nonavalent HPV Vaccine. Pediatrics. 2023:e2022060301.
Akther T, Nur T. A model of factors influencing COVID-19 vaccine acceptance: A synthesis of the theory of reasoned action, conspiracy theory belief, awareness, perceived usefulness, and perceived ease of use. PLOS ONE. 2022;17(1):e0261869.
Kreimer AR, Herrero R, Sampson JN, Porras C, Lowy DR, Schiller JT, et al. Evidence for single-dose protection by the bivalent HPV vaccine—review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. 2018;36(32):4774-82.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R: A hands-on guide: CRC Press; 2021.
IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247.
Langan D, Higgins JP, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, et al. A comparison of heterogeneity variance estimators in simulated random‐effects meta-analyses. Res Synth Methods. 2019;10(1):83-98.
Joanna I, John PAI, Maroeska MR, Jelle JG. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247.
Minozzi S, Cinquini M, Gianola S, Gonzalez-Lorenzo M, Banzi R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol. 2020;126:37-44.
Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. 2011;2(1):1-12.
Setiawan D, Nurulita NA, Mukaromah S, Postma MJ. The Clinical Effectiveness of One-Dose HPV Vaccine: A meta-analysis of 902,368 vaccinated women. medRxiv. 2023:2023.08. 17.23294214.
Reyburn R, Tuivaga E, Ratu T, Young S, Garland SM, Murray G, et al. A single dose of quadrivalent HPV vaccine is highly effective against HPV genotypes 16 and 18 detection in young pregnant women eight years following vaccination: an retrospective cohort study in Fiji. Lancet Reg Health–West Pac. 2023;37.
Kavanagh K, Pollock KG, Cuschieri K, Palmer T, Cameron RL, Watt C, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis. 2017;17(12):1293-302.
Waheed DE, Burdier FR, Eklund C, Baussano I, Mariz FC, Téblick L, et al. An update on one-dose HPV vaccine studies, immunobridging and humoral immune responses - A meeting report. Prev Med Rep. 2023;35:102368.
Barnabas RV, Brown ER, Onono MA, Bukusi EA, Njoroge B, Winer RL, et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evid. 2022;1(5):EVIDoa2100056.
Ebrahimi N, Yousefi Z, Khosravi G, Malayeri FE, Golabi M, Askarzadeh M, et al. Human papillomavirus vaccination in low- and middle-income countries: progression, barriers, and future prospective. Front Immunol. 2023;14:1150238.
Prem K, Choi YH, Bénard É, Burger EA, Hadley L, Laprise JF, et al. Global impact and cost-effectiveness of one-dose versus two-dose human papillomavirus vaccination schedules: a comparative modelling analysis. BMC Med. 2023;21(1):313.
Organization WH. One-dose Human Papillomavirus (HPV) vaccine offers solid protection against cervical cancer. Geneva: World Health Organization. 2022.
Whitworth HS, Gallagher KE, Howard N, Mounier-Jack S, Mbwanji G, Kreimer AR, et al. Efficacy and immunogenicity of a single dose of human papillomavirus vaccine compared to no vaccination or standard three and two-dose vaccination regimens: a systematic review of evidence from clinical trials. Vaccine. 2020;38(6):1302-14.
Zou Z, Zhang L. The one-dose schedule opens the door to rapid scale-up of HPV vaccination. BMC Med. 2023;21(1):387.
How to Cite
License
Copyright (c) 2024 Shibaji Gupta, Srija Basu, Rudradeep Banerjee

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright© by the author(s). Published by the Evidence Journals. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.