Abstract
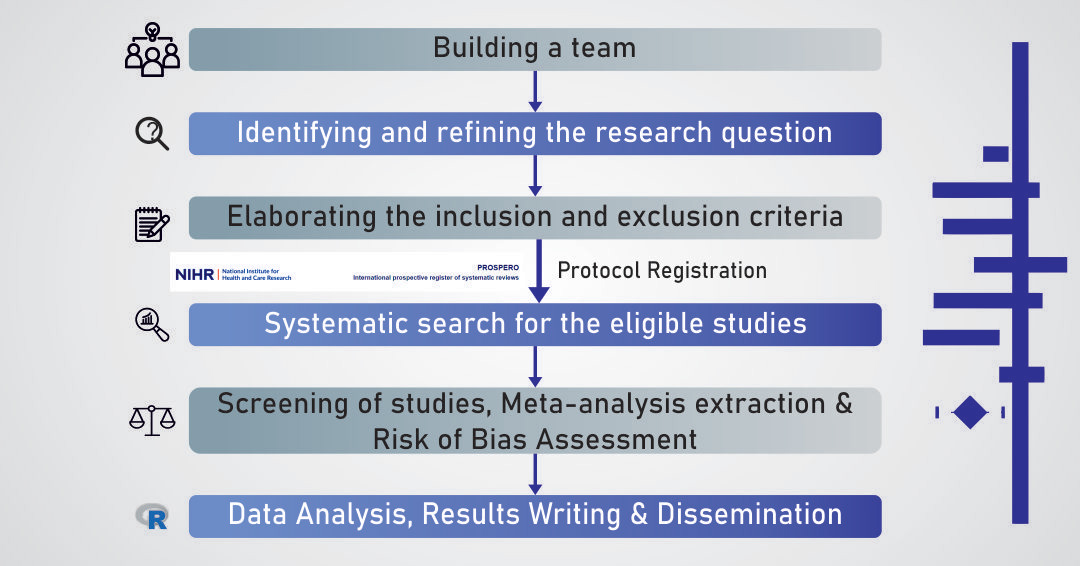
Evidence-based research is used to generate, summarise, and understand the best available practices to inform decision-making. Systematic reviews (SR) and meta-analysis (MA) have become a valuable tool for these goals in public health, medicine and pharmaceutical research. MA is a statistical procedure for combining the results of multiple studies investigating a common intervention or issue to produce a pooled effect size and evaluate interventions' efficacy across studies. This article outlines the usefulness of systematic reviews and meta-analysis in medicine and public health. Steps in undertaking the systematic reviews and meta-analysis include forming a team, identifying and refining the research question, determining the inclusion and exclusion criteria, registering the SR and MA protocol, searching for the studies, selection of the studies, data extraction, data analysis and presenting the results. The review also outlines the issues that can impact meaningful meta-analysis. The heterogeneity in the included studies, conditions studied, interventions, and end-point measures is one of the major issues encountered in meta-analysis. Quantification of the heterogeneity can be done by I2 statistics and prediction intervals. Sub-group analysis, outlier detection followed by sensitivity analysis, and meta-regression can be applied to explore and reduce heterogeneity. Random effects model, Knapp-Hartung, likelihood estimates, and Bayesian models can be applied in a highly heterogenous meta-analysis.
Keywords:
Meta-analysis, Systematic review, Heterogeneity, Evidence based medicine, Evidence based public health, Evidence synthesis, Sub-group analysis, Meta-regression, Prediction intervalReferences
Thoma A, Eaves FF. A Brief History of Evidence-Based Medicine (EBM) and the Contributions of Dr David Sackett. Aesthetic Surg J [Internet]. 2015 Nov 1 [cited 2023 Jan 5];35(8):NP261–3. Available from: https://academic.oup.com/asj/article/35/8/NP261/251339
Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. Vol. 312, BMJ (Clinical research ed.). England; 1996. p. 71–2.
Mikolajewicz N, Komarova S V. Meta-analytic methodology for basic research: A practical guide. Front Physiol. 2019 Mar 27;10(MAR):203.
Lhachimi SK, Bala MM, Vanagas G. Evidence-Based Public Health. Biomed Res Int [Internet]. 2016 [cited 2023 Jan 5];2016. Available from: /pmc/articles/PMC4749765/
Tenny S, Varacallo M. Evidence Based Medicine. StatPearls [Internet]. 2022 Oct 24 [cited 2023 Jan 5]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK470182/
Song JW, Chung KC. Observational Studies: Cohort and Case-Control Studies. Plast Reconstr Surg [Internet]. 2010 Dec [cited 2023 Jan 18];126(6):2234. Available from: /pmc/articles/PMC2998589/
Kang H. Use, application, and interpretation of systematic reviews and meta-analyses. Korean J Anesthesiol [Internet]. 2021 Oct 1 [cited 2023 Jan 18];74(5):369. Available from: /pmc/articles/PMC8497910/
Methods - Application of Systematic Review Methodology to the Field of Nutrition - NCBI Bookshelf [Internet]. [cited 2023 Jan 18]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK44075/
Glass G V. Primary, Secondary, and Meta-Analysis of Research. Educ Res. 1976 Nov;5(10):3.
Paul M, Leibovici L. Systematic review or meta-analysis? Their place in the evidence hierarchy. Vol. 20, Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. England; 2014. p. 97–100.
Ahn E, Kang H. Introduction to systematic review and meta-analysis. Korean J Anesthesiol. 2018 Apr;71(2):103–12.
Kang H. Statistical considerations in meta-analysis. Hanyang Med Rev. 2015;35(1):23–32.
George BJ, Aban IB. An application of meta-analysis based on DerSimonian and Laird method [Internet]. Journal of Nuclear Cardiology. Springer; 2016. Available from: https://link.springer.com/article/10.1007/s12350-015-0249-6
Haidich AB. Meta-analysis in medical research. Hippokratia [Internet]. 2010 [cited 2023 Jan 6];14(Suppl 1):29. Available from: /pmc/articles/PMC3049418/
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000 Apr;283(15):2008–12.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ [Internet]. 2021 Mar [cited 2021 Dec 27];372:n71. Available from: https://www.bmj.com/content/372/bmj.n71
Tawfik GM, Dila KAS, Mohamed MYF, Tam DNH, Kien ND, Ahmed AM, et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health. 2019;47:46.
Uman LS. Systematic Reviews and Meta-Analyses. J Can Acad Child Adolesc Psychiatry [Internet]. 2011 [cited 2023 Jan 18];20(1):57. Available from: /pmc/articles/PMC3024725/
Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA Annu Symp Proc [Internet]. 2006 [cited 2023 Jan 18];2006:359. Available from: /pmc/articles/PMC1839740/
Abbade LPF, Wang M, Sriganesh K, Mbuagbaw L, Thabane L. Framing of research question using the PICOT format in randomised controlled trials of venous ulcer disease: a protocol for a systematic survey of the literature. BMJ Open [Internet]. 2016 Nov 1;6(11):e013175. Available from: http://bmjopen.bmj.com/content/6/11/e013175.abstract
El Dib R, Periyasamy AG, de Barros JL, França CG, Senefonte FL, Vesentini G, et al. Probiotics for the treatment of depression and anxiety: A systematic review and meta-analysis of randomised controlled trials. Clin Nutr ESPEN. 2021 Oct 1;45:75–90.
Sahoo S, Yadav S, Shamim MA, Nisha S, Sandeep M, Padhi BK, et al. Prevalence of suicidal behavior in adolescents in India: A Systematic review and Meta-analysis. Asian J Psychiatr [Internet]. 2023;86(August):103661. Available from: https://www.sciencedirect.com/science/article/pii/S1876201823002174
Gandhi AP, Gupta PC, Padhi BK, Sandeep M, Suvvari TK, Shamim MA, et al. Ophthalmic Manifestations of the Monkeypox Virus: A Systematic Review and Meta-Analysis. Vol. 12, Pathogens. 2023.
Gandhi P A, Patro SK, Sandeep M, Satapathy P, Shamim MA, Kumar V, et al. Oral manifestation of the monkeypox virus: a systematic review and meta-analysis. eClinicalMedicine [Internet]. 2023 Feb 1 [cited 2023 Jan 9];56:101817. Available from: http://www.thelancet.com/article/S2589537022005466/fulltext
Pieper D, Rombey T. Where to prospectively register a systematic review. Syst Rev [Internet]. 2022;11(1):8. Available from: https://doi.org/10.1186/s13643-021-01877-1
Systematic Review Protocols and Protocol Registries | NIH Library [Internet]. [cited 2023 Jun 16]. Available from: https://www.nihlibrary.nih.gov/services/systematic-review-service/systematic-review-protocols-and-protocol-registries
PROSPERO [Internet]. [cited 2023 Jan 6]. Available from: https://www.crd.york.ac.uk/prospero/
Booth A, Stewart L. Trusting researchers to use open trial registers such as PROSPERO responsibly. Vol. 347, BMJ (Clinical research ed.). England; 2013. p. f5870.
PubMed [Internet]. [cited 2023 Jan 18]. Available from: https://pubmed.ncbi.nlm.nih.gov/
Welcome - Embase [Internet]. [cited 2023 Jan 18]. Available from: https://www.embase.com/landing?status=yellow
ProQuest | Better research, better learning, better insights. [Internet]. [cited 2023 Jan 18]. Available from: https://www.proquest.com/
EBSCOhost Research Platform | EBSCO [Internet]. [cited 2023 Jan 18]. Available from: https://www.ebsco.com/products/ebscohost-research-platform
CINAHL Complete | EBSCO [Internet]. [cited 2023 Jan 18]. Available from: https://www.ebsco.com/products/research-databases/cinahl-complete
Scopus preview - Scopus - Welcome to Scopus [Internet]. [cited 2023 Jan 18]. Available from: https://www.scopus.com/
Ovid: Welcome to Ovid [Internet]. [cited 2023 Jan 18]. Available from: https://ovidsp.ovid.com/
Clarivate [Internet]. [cited 2023 Jan 18]. Available from: https://access.clarivate.com/login?app=wos&alternative=true&shibShireURL=https:%2F%2Fwww.webofknowledge.com%2F%3Fauth%3DShibboleth&shibReturnURL=https:%2F%2Fwww.webofknowledge.com%2F&roaming=true
APA PsycInfo [Internet]. [cited 2023 Jan 18]. Available from: https://www.apa.org/pubs/databases/psycinfo
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017 Sep;358:j4008.
arXiv.org e-Print archive [Internet]. [cited 2023 Jan 18]. Available from: https://arxiv.org/
Homepage | ChemRxiv | Cambridge Open Engage [Internet]. [cited 2023 Jan 18]. Available from: https://chemrxiv.org/engage/chemrxiv/public-dashboard
Home :: SSRN [Internet]. [cited 2023 Jan 18]. Available from: https://www.ssrn.com/index.cfm/en/
medRxiv.org - the pre-print server for Health Sciences [Internet]. [cited 2023 Jan 18]. Available from: https://www.medrxiv.org/
bioRxiv.org - the pre-print server for Biology [Internet]. [cited 2023 Jan 18]. Available from: https://www.biorxiv.org/
Handsearching - Systematic Reviews in the Health Sciences - Research Guides at Rutgers University [Internet]. [cited 2023 Jan 18]. Available from: https://libguides.rutgers.edu/c.php?g=337288&p=2269575
Vassar M, Atakpo P, Kash MJ. Manual search approaches used by systematic reviewers in dermatology. J Med Libr Assoc [Internet]. 2016 Jan 5 [cited 2023 Jan 18];104(4):302–304–302–304. Available from: https://jmla.pitt.edu/ojs/jmla/article/view/145
Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev [Internet]. 2017;6(1):245. Available from: https://doi.org/10.1186/s13643-017-0644-y
Rathbone J, Carter M, Hoffmann T, Glasziou P. Better duplicate detection for systematic reviewers: evaluation of Systematic Review Assistant-Deduplication Module. Syst Rev [Internet]. 2015;4(1):6. Available from: https://doi.org/10.1186/2046-4053-4-6
Deduplicating - Systematic Reviews, Scoping Reviews, and other Knowledge Syntheses - Guides at McGill Library [Internet]. [cited 2023 Jan 18]. Available from: https://libraryguides.mcgill.ca/knowledge-syntheses/deduplicating
Etchells E. Ovid. BMJ Evidence-Based Med [Internet]. 2000;5(3):70. Available from: https://ebm.bmj.com/content/5/3/70
Ovid is the world's most trusted medical research platform | Wolters Kluwer [Internet]. [cited 2023 Jun 19]. Available from: https://www.wolterskluwer.com/en-in/solutions/ovid
Waffenschmidt S, Knelangen M, Sieben W, Bühn S, Pieper D. Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Med Res Methodol [Internet]. 2019;19(1):132. Available from: https://doi.org/10.1186/s12874-019-0782-0
Polanin JR, Pigott TD, Espelage DL, Grotpeter JK. Best practice guidelines for abstract screening large‐evidence systematic reviews and meta‐analyses. Res Synth Methods [Internet]. 2019 Sep 1 [cited 2023 Jan 18];10(3):330. Available from: /pmc/articles/PMC6771536/
Covidence - Better systematic review management [Internet]. [cited 2023 Jan 6]. Available from: https://www.covidence.org/
Rayyan – Intelligent Systematic Review - Rayyan [Internet]. [cited 2023 Jan 6]. Available from: https://www.rayyan.ai/
Nested Knowledge – Transforming the systematic review and meta-analysis paradigm from static out-of-date PDFs to dynamic living, interactive web-based visuals. [Internet]. [cited 2023 Jun 19]. Available from: https://about.nested-knowledge.com/
Marshall IJ, Wallace BC. Toward systematic review automation: a practical guide to using machine learning tools in research synthesis. [Internet]. Systematic reviews England: BioMed Central; Jul 11, 2019 p. 163. Available from: /pmc/articles/PMC6621996/
Marshall IJ, Kuiper J, Wallace BC. RobotReviewer: evaluation of a system for automatically assessing bias in clinical trials. J Am Med Inform Assoc. 2016 Jan;23(1):193–201.
Marshall IJ, Noel-Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying Randomized Controlled Trials: An evaluation and practitioner's guide. Res Synth Methods. 2018 Dec;9(4):602–14.
Wallace BC, Noel-Storr A, Marshall IJ, Cohen AM, Smalheiser NR, Thomas J. Identifying reports of randomised controlled trials (RCTs) via a hybrid machine learning and crowdsourcing approach. J Am Med Inform Assoc. 2017 Nov;24(6):1165–8.
Soto AJ, Przybyła P, Ananiadou S. Thalia: semantic search engine for biomedical abstracts. Bioinformatics. 2019 May;35(10):1799–801.
Mo Y, Kontonatsios G, Ananiadou S. Supporting systematic reviews using LDA-based document representations. Syst Rev. 2015 Nov;4:172.
Cohen AM, Smalheiser NR, McDonagh MS, Yu C, Adams CE, Davis JM, et al. Automated confidence ranked classification of randomised controlled trial articles: an aid to evidence-based medicine. J Am Med Inform Assoc. 2015 May;22(3):707–17.
Saldanha IJ, Lindsley KB, Money S, Kimmel HJ, Smith BT, Dickersin K. Outcome choice and definition in systematic reviews leads to few eligible studies included in meta-analyses: a case study. BMC Med Res Methodol [Internet]. 2020;20(1):30. Available from: https://doi.org/10.1186/s12874-020-0898-2
Chapter 10: Analysing data and undertaking meta-analyses | Cochrane Training [Internet]. [cited 2023 Jan 6]. Available from: https://training.cochrane.org/handbook/current/chapter-10
Riley RD, Sutton AJ, Abrams KR, Lambert PC. Sensitivity analyses allowed more appropriate and reliable meta-analysis conclusions for multiple outcomes when missing data was present. J Clin Epidemiol [Internet]. 2004 Sep 1 [cited 2023 Jan 18];57(9):911–24. Available from: http://www.jclinepi.com/article/S0895435604001143/fulltext
Marušić MF, Fidahić M, Cepeha CM, Farcaș LG, Tseke A, Puljak L. Methodological tools and sensitivity analysis for assessing quality or risk of bias used in systematic reviews published in the high-impact anesthesiology journals. BMC Med Res Methodol [Internet]. 2020;20(1):121. Available from: https://doi.org/10.1186/s12874-020-00966-4
Kennedy CE, Fonner VA, Armstrong KA, Denison JA, Yeh PT, O'Reilly KR, et al. The Evidence Project risk of bias tool: assessing study rigor for both randomised and non-randomised intervention studies. Syst Rev [Internet]. 2019;8(1):3. Available from: https://doi.org/10.1186/s13643-018-0925-0
Frampton G, Whaley P, Bennett M, Bilotta G, Dorne J-LCM, Eales J, et al. Principles and framework for assessing the risk of bias for studies included in comparative quantitative environmental systematic reviews. Environ Evid [Internet]. 2022;11(1):12. Available from: https://doi.org/10.1186/s13750-022-00264-0
Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018 Sep;74(3):785–94.
Sedgwick P. What is publication bias in a meta-analysis? BMJ [Internet]. 2015 Aug 14 [cited 2023 Jan 18];351. Available from: https://www.bmj.com/content/351/bmj.h4419
Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol [Internet]. 2000;53(2):207–16. Available from: https://www.sciencedirect.com/science/article/pii/S0895435699001614
Mathur MB, VanderWeele TJ. Sensitivity analysis for publication bias in meta-analyses. J R Stat Soc Ser C Appl Stat. 2020 Nov;69(5):1091–119.
Borenstein M, Higgins JPT. Meta-analysis and subgroups. Prev Sci. 2013 Apr;14(2):134–43.
Gandhi P., Satapathy P, Sarvesh R, Hermis AH, Sah R, Padhi BK. Comments on "Shigellosis in Southeast Asia: A systematic review and meta-analysis." Travel Med Infect Dis. 2023;54:102593.
Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc [Internet]. 2018;16(4):195–203. Available from: https://journals.lww.com/ijebh/fulltext/2018/12000/a_new_improved_graphical_and_quantitative_method.3.aspx
What is GRADE? | BMJ Best Practice [Internet]. [cited 2023 Jan 6]. Available from: https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/
Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med [Internet]. 2017 Jun 1 [cited 2023 Jan 18];22(3):85. Available from: /pmc/articles/PMC5502230/
Assess Certainty - Systematic Reviews and Meta-Analyses - Research Guides at Virginia Tech [Internet]. [cited 2023 Jan 18]. Available from: https://guides.lib.vt.edu/SRMA/assesscertainty
Assessing certainty of evidence | NHMRC [Internet]. [cited 2023 Jan 18]. Available from: https://www.nhmrc.gov.au/guidelinesforguidelines/develop/assessing-certainty-evidence
Forest Plot - an overview | ScienceDirect Topics [Internet]. [cited 2023 Jan 18]. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/forest-plot
Dettori JR, Norvell DC, Chapman JR. Seeing the Forest by Looking at the Trees: How to Interpret a Meta-AnalysisForest Plot. Glob Spine J [Internet]. 2021 May 1 [cited 2023 Jan 18];11(4):614. Available from: /pmc/articles/PMC8119923/
Funnel Plot - an overview | ScienceDirect Topics [Internet]. [cited 2023 Jan 18]. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/funnel-plot
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ [Internet]. 2011 Jul 22 [cited 2023 Jan 18];343(7818). Available from: https://www.bmj.com/content/343/bmj.d4002
Siddaway AP, Wood AM, Hedges L V. How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-Analyses, and Meta-Syntheses. Annu Rev Psychol. 2019 Jan;70:747–70.
Greco T, Zangrillo A, Biondi-Zoccai G, Landoni G. Meta-analysis: pitfalls and hints. Hear Lung Vessel [Internet]. 2013 [cited 2023 Jan 6];5(4):219. Available from: /pmc/articles/PMC3868184/
Veginadu P, Calache H, Gussy M, Pandian A, Masood M. An overview of methodological approaches in systematic reviews. J Evid Based Med [Internet]. 2022 Mar 1 [cited 2023 Jan 6];15(1):39–54. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/jebm.12468
Gandhi PA, Patro SK, Sandeep M, Satapathy S, Shamim MA, Kumar V, et al. Oral manifestation of the monkeypox virus: A systematic review and meta-analysis. eClinicalMedicine [Internet]. 2023;56:101817. Available from: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(22)00546-6/fulltext
Murad MH, Montori VM, Ioannidis JPA, Jaeschke R, Devereaux PJ, Prasad K, et al. How to Read a Systematic Review and Meta-analysis and Apply the Results to Patient Care: Users' Guides to the Medical Literature. JAMA [Internet]. 2014;312(2):171–9. Available from: https://doi.org/10.1001/jama.2014.5559
Fletcher J. Clinical Epidemiology Notes: What is heterogeneity and is it important? BMJ Br Med J [Internet]. 2007 Jan 1 [cited 2023 Jan 18];334(7584):94. Available from: /pmc/articles/PMC1767262/
Sandercock P. The Authors Say: 'The Data Are Not So Robust because of Heterogeneity' – So, How Should I Deal with This Systematic Review? Cerebrovasc Dis [Internet]. 2011 May [cited 2023 Jan 18];31(6):615–20. Available from: https://www.karger.com/Article/FullText/326068
Imrey PB. Limitations of Meta-analyses of Studies With High Heterogeneity. JAMA Netw Open [Internet]. 2020;3(1):e1919325–e1919325. Available from: https://doi.org/10.1001/jamanetworkopen.2019.19325
Schroll JB, Moustgaard R, Gøtzsche PC. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med Res Methodol [Internet]. 2011 Feb [cited 2023 Jan 18];11:22. Available from: /pmc/articles/PMC3056846/
Ruppar T. Meta-analysis: How to quantify and explain heterogeneity? Eur J Cardiovasc Nurs. 2020 Oct;19(7):646–52.
Spineli LM, Pandis N. Exploring heterogeneity in meta-analysis: Meta-regression analysis. Am J Orthod Dentofac Orthop [Internet]. 2020 Oct 1 [cited 2023 Jan 18];158(4):623–5. Available from: http://www.ajodo.org/article/S088954062030408X/fulltext
Jansen JP. Heterogeneity and Subgroup Analysis in Network Meta-Analysis BT - Design and Analysis of Subgroups with Biopharmaceutical Applications. In: Ting N, Cappelleri JC, Ho S, Chen (Din) Ding-Geng, editors. Cham: Springer International Publishing; 2020. p. 369–85. Available from: https://doi.org/10.1007/978-3-030-40105-4_18
Sedgwick P. Meta-analyses: what is heterogeneity? BMJ [Internet]. 2015;350. Available from: https://www.bmj.com/content/350/bmj.h1435
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun;21(11):1539–58.
Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods [Internet]. 2010 Apr;1(2):112–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26061377
Willis BH, Riley RD. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat Med. 2017 Sep;36(21):3283–301.
Baujat B, Mahé C, Pignon J-P, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. 2002 Sep;21(18):2641–52.
Bowden J, Spiller W, Del Greco M F, Sheehan N, Thompson J, Minelli C, et al. Improving the visualisation, interpretation and analysis of two-sample summary data Mendelian randomisation via the Radial plot and Radial regression. Int J Epidemiol. 2018 Aug;47(4):1264–78.
Matsushima Y, Noma H, Yamada T, Furukawa TA. Influence diagnostics and outlier detection for meta-analysis of diagnostic test accuracy. Res Synth Methods. 2020 Mar;11(2):237–47.
Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract. 2009 Oct;63(10):1426–34.
Introduction - Meta-regression Approaches - NCBI Bookshelf [Internet]. [cited 2023 Jan 18]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK43897/
Serghiou S, Goodman SN. Random-Effects Meta-analysis: Summarising Evidence With Caveats. JAMA [Internet]. 2019;321(3):301–2. Available from: https://doi.org/10.1001/jama.2018.19684
Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014 Feb;160(4):267–70.
Aert RCM Van, Jackson D. A new justification of the Hartung‐Knapp method for random‐effects meta‐analysis based on weighted least squares regression. Res Synth Methods [Internet]. 2019; Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/jrsm.1356
IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol [Internet]. 2014;14(1):25. Available from: https://doi.org/10.1186/1471-2288-14-25
Hackenberger BK. Bayesian meta-analysis now–let's do it. Croat Med J. 2020;61(6):564.
IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open [Internet]. 2016;6(7). Available from: https://bmjopen.bmj.com/content/6/7/e010247
Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009 Jan;172(1):137–59.
How to Cite
License
Copyright (c) 2023 The Evidence

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright© by the author(s). Published by the Evidence Journals. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Most read articles by the same author(s)
- Muhammad Aaqib Shamim, Aravind P Gandhi, Pradeep Dwivedi, Bijaya Kumar Padhi, How to perform meta-analysis in R: a simple yet comprehensive guide , The Evidence: Vol. 1 No. 1 (2023): OCT - DEC
- Russell Kabir, Haniya Zehra Syed, Richard Hayhoe, Ali Davod Parsa, Madhini Sivasubramanian, Masoud Mohammadnezhad, Brijesh Sathian, Kizhessery Rahna, Manav Jain, Aravind P Gandhi, Muhammad Aaqib Shamim, Shoban Babu Varthya, Surjit Singh, Pradeep Dwivedi, Meta-analysis using SPSS: a simple guide for clinicians, public health, and allied health specialists , The Evidence: Vol. 2 No. 1 (2024): JAN - MAR
- Aravind P Gandhi, Pradeep Deshmukh, Amol R Dongre, Qualitative evidence synthesis: applications, methods, challenges, and opportunities , The Evidence: Vol. 3 No. 1 (2025): JAN - MAR