Abstract
Background: Cardiovascular disease (CVD) is a major global health challenge, with increasing prevalence despite advancements in treatment. Recently, the gut microbiota's role in human metabolism, immunity, and disease processes, including CVD, has gained significant attention.
Objectives: This review seeks to elucidate the relationship between gut microorganisms and the development and progression of CVD.
Method: A comprehensive review was conducted, focusing on the significant microorganisms associated with CVD, the mechanisms through which the gut microbiome influences CVD, and the diagnostic modalities used to detect these microorganisms.
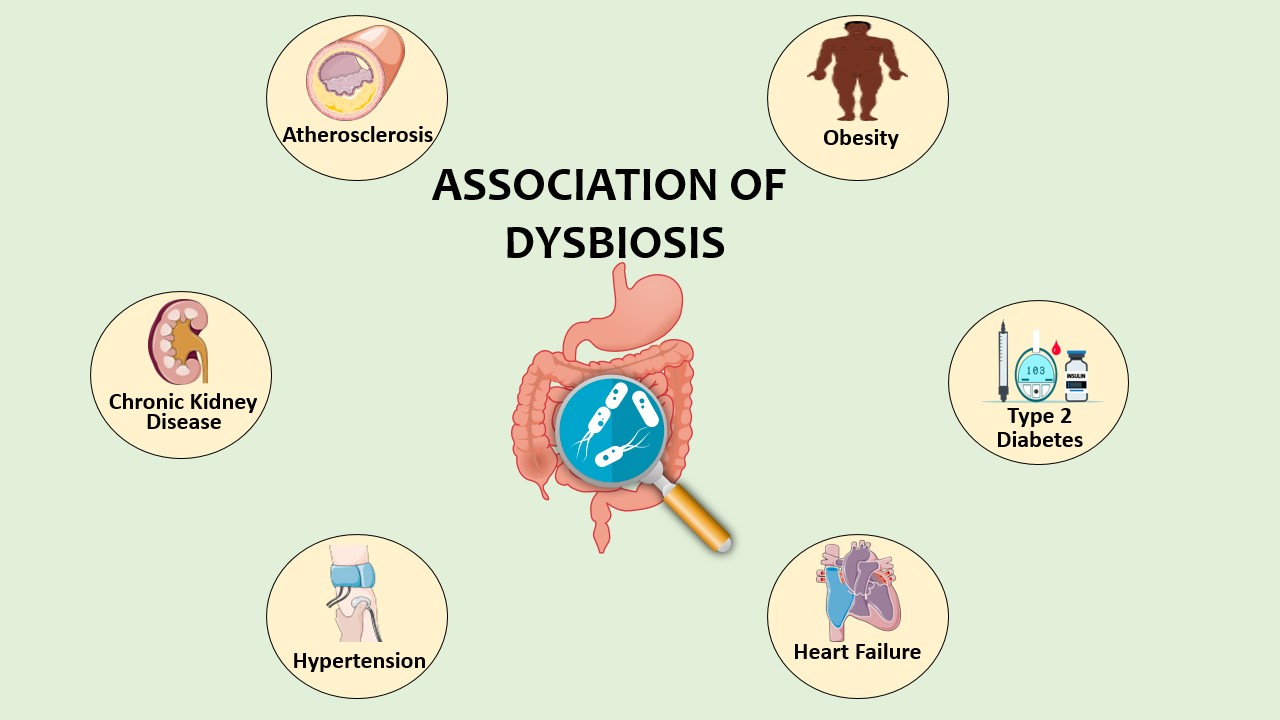
Results: CVD can arise from various infectious and non-infectious agents, with certain microorganisms being implicated in heart failure, atherosclerosis, and other cardiovascular conditions. Dysbiosis, or disruption of the gut microbiota, has been linked to increased inflammation and the development of atherosclerosis. Advanced molecular biology tools, such as PCR and next-generation sequencing, have proven effective in detecting microbial pathogens associated with CVD. The gut microbiome's interaction with the host occurs through various pathways, and disruptions in its composition or metabolites can contribute to CVD risks.
Conclusion: The gut microbiota plays a pivotal role in modulating systemic immune responses and metabolic dysfunctions, contributing to CVD development. Understanding this relationship offers potential therapeutic targets and strategies for preventing and treating CVD. Future research should focus on specific microbial strains, microbiome-mediated metabolites, and personalized interventions to harness the gut microbiota's therapeutic potential.
Keywords:
Cardiovascular disease (CVD), Gut microbiota, Dysbiosis, Atherosclerosis, Inflammation, Microbial pathogens, Diagnostic modalities, Next-generation sequencing (NGS), Metabolites, Therapeutic interventionsReferences
Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12): e493]. Circulation. 2018;137(12): e67-e492. doi:10.1161/CIR.0000000000000558
Muller AM, Fischer A, Katus HA, Kaya Z. Mouse models of autoimmune diseases - autoimmune myocarditis. Curr Pharm Des. 2015;21(18):2498-2512. doi:10.2174/1381612821666150316123711
Fournier PE, Gouriet F, Casalta JP, et al. Blood culture-negative endocarditis: Improving the diagnostic yield using new diagnostic tools. Medicine (Baltimore). 2017;96(47):e8392. doi:10.1097/MD.0000000000008392
Boudebouch N, Sarih M, Chakib A, et al. Blood Culture-Negative Endocarditis, Morocco. Emerg Infect Dis. 2017;23(11):1908-1909. doi:10.3201/eid2311.161066
Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev. 2001;14(1):177-207. doi:10.1128/CMR.14.1.177-207.2001
Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96(11):4095-4103. doi:10.1161/01.cir.96.11.4095
Mitra, S., Drautz-Moses, D.I., Alhede, M. et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 3, 38 (2015). https://doi.org/10.1186/s40168-015-0100-y
Chistiakov DA, Bobryshev YV, Kozarov E, Sobenin IA, Orekhov AN. Role of gut microbiota in the modulation of atherosclerosis-associated immune response. Front Microbiol. 2015;6:671. Published 2015 Jun 30. doi:10.3389/fmicb.2015.00671
Kim S, Goel R, Kumar A, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond). 2018;132(6):701-718. Published 2018 Mar 30. doi:10.1042/CS20180087
Karbach SH, Schönfelder T, Brandão I, et al. Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. J Am Heart Assoc. 2016;5(9):e003698. Published 2016 Aug 30. doi:10.1161/JAHA.116.003698
Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120(7):1183-1196. doi:10.1161/CIRCRESAHA.117.309715
Yin J, Liao SX, He Y, et al. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J Am Heart Assoc. 2015;4(11):e002699. Published 2015 Nov 23. doi:10.1161/JAHA.115.002699
Fu J, Bonder MJ, Cenit MC, et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ Res. 2015;117(9):817-824. doi:10.1161/CIRCRESAHA.115.306807
Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258-1270. doi:10.1016/j.cell.2012.01.035
Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242-249. doi:10.1038/nature11552
Yamashiro K, Tanaka R, Urabe T, et al. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke [published correction appears in PLoS One. 2017 Apr 13;12 (4):e0176062]. PLoS One. 2017;12(2):e0171521. Published 2017 Feb 6. doi:10.1371/journal.pone.0171521
Barin JG, Talor MV, Diny NL, et al. Regulation of autoimmune myocarditis by host responses to the microbiome. Exp Mol Pathol. 2017;103(2):141-152. doi:10.1016/j.yexmp.2017.08.003
Tripathi A, Xu ZZ, Xue J, et al. Intermittent Hypoxia and Hypercapnia Reproducibly Change the Gut Microbiome and Metabolome across Rodent Model Systems. mSystems. 2019;4(2):e00058-19. Published 2019 Apr 30. doi:10.1128/mSystems.00058-19
Xu M, Liu PP, Li H. Innate Immune Signaling and Its Role in Metabolic and Cardiovascular Diseases. Physiol Rev. 2019;99(1):893-948. doi:10.1152/physrev.00065.2017
Adamczyk-Sowa M, Medrek A, Madej P, Michlicka W, Dobrakowski P. Does the Gut Microbiota Influence Immunity and Inflammation in Multiple Sclerosis Pathophysiology?. J Immunol Res. 2017;2017:7904821. doi:10.1155/2017/7904821
Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120(7):1183-1196. doi:10.1161/CIRCRESAHA.117.309715
Levy M, Thaiss CA, Zeevi D, et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell. 2015;163(6):1428-1443. doi:10.1016/j.cell.2015.10.048
Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268-1273. doi:10.1126/science.1223490
Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718-15723. doi:10.1073/pnas.0407076101
Gabriel CL, Ferguson JF. Gut Microbiota and Microbial Metabolism in Early Risk of Cardiometabolic Disease. Circ Res. 2023;132(12):1674-1691. doi:10.1161/CIRCRESAHA.123.322055
Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5(1):80. Published 2017 Jul 14. doi:10.1186/s40168-017-0296-0
Schiattarella GG, Sannino A, Esposito G, Perrino C. Diagnostics and therapeutic implications of gut microbiota alterations in cardiometabolic diseases. Trends Cardiovasc Med. 2019;29(3):141-147. doi:10.1016/j.tcm.2018.08.003
Serino M, Blasco-Baque V, Nicolas S, Burcelin R. Far from the eyes, close to the heart: dysbiosis of gut microbiota and cardiovascular consequences. Curr Cardiol Rep. 2014;16(11):540. doi:10.1007/s11886-014-0540-1
Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59-65. doi:10.1038/nature08821
Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness [published correction appears in Nature. 2013 Oct 24;502(7472)580]. Nature. 2013;500(7464):585-588. doi:10.1038/nature12480
Bullon P, Cordero MD, Quiles JL, Morillo JM, del Carmen Ramirez-Tortosa M, Battino M. Mitochondrial dysfunction promoted by Porphyromonas gingivalis lipopolysaccharide as a possible link between cardiovascular disease and periodontitis. Free Radic Biol Med. 2011;50(10):1336-1343. doi:10.1016/j.freeradbiomed.2011.02.018
Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47(4):333-343. doi:10.1016/j.freeradbiomed.2009.05.00433.
Ramirez-Tortosa MC, Quiles JL, Battino M, et al. Periodontitis is associated with altered plasma fatty acids and cardiovascular risk markers. Nutr Metab Cardiovasc Dis. 2010;20(2):133-139. doi:10.1016/j.numecd.2009.03.003
Kuo LC, Polson AM, Kang T. Associations between periodontal diseases and systemic diseases: a review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health. 2008;122(4):417-433. doi:10.1016/j.puhe.2007.07.004
Gan XT, Ettinger G, Huang CX, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7(3):491-499. doi:10.1161/CIRCHEARTFAILURE.113.000978
Shi L, Ji Y, Zhao S, et al. Crosstalk between reactive oxygen species and Dynamin-related protein 1 in periodontitis. Free Radic Biol Med. 2021;172:19-32. doi:10.1016/j.freeradbiomed.2021.05.031
Zaja I, Bai X, Liu Y, et al. Cdk1, PKCδ and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem Biophys Res Commun. 2014;453(4):710-721. doi:10.1016/j.bbrc.2014.09.144
Barin JG, Tobias LD, Peterson DA. The microbiome and autoimmune disease: Report from a Noel R. Rose Colloquium. Clin Immunol. 2015;159(2):183-188. doi:10.1016/j.clim.2015.05.009
Barin JG, Čiháková D. Control of inflammatory heart disease by CD4+ T cells. Ann N Y Acad Sci. 2013;1285:80-96. doi:10.1111/nyas.12134
De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1-2):84-96. doi:10.1016/j.cell.2013.12.016
Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18(1):190-195. doi:10.1038/oby.2009.167
Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364-371. doi:10.2337/db11-1019
Baban B, Yin L, Qin X, Liu JY, Shi X, Mozaffari MS. The role of GILZ in modulation of adaptive immunity in a murine model of myocardial infarction. Exp Mol Pathol. 2017;102(3):408-414. doi:10.1016/j.yexmp.2017.05.002
McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog Lipid Res. 2011;50(4):331-347. doi:10.1016/j.plipres.2011.04.002
Brouqui P, Raoult D. New insight into the diagnosis of fastidious bacterial endocarditis. FEMS Immunol Med Microbiol. 2006;47(1):1-13. doi:10.1111/j.1574-695X.2006.00054.x
Brinkman CL, Vergidis P, Uhl JR, et al. PCR-electrospray ionization mass spectrometry for direct detection of pathogens and antimicrobial resistance from heart valves in patients with infective endocarditis. J Clin Microbiol. 2013;51(7):2040-2046. doi:10.1128/JCM.00304-13
Hasman H, Saputra D, Sicheritz-Ponten T, et al. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples [published correction appears in J Clin Microbiol. 2014 Aug;52(8):3136]. J Clin Microbiol. 2014;52(1):139-146. doi:10.1128/JCM.02452-13
Cheng J, Hu H, Fang W, et al. Detection of pathogens from resected heart valves of patients with infective endocarditis by next-generation sequencing. Int J Infect Dis. 2019;83:148-153. doi:10.1016/j.ijid.2019.03.007
Peeters B, Herijgers P, Beuselinck K, et al. Added diagnostic value and impact on antimicrobial therapy of 16S rRNA PCR and amplicon sequencing on resected heart valves in infective endocarditis: a prospective cohort study. Clin Microbiol Infect. 2017;23(11):888.e1-888.e5. doi:10.1016/j.cmi.2017.06.008
Mohanty, A., Singh T, S., Kabi, A., Gupta, P., Gupta, P., & Kumar, P. (2017, November 1). BACTERIOLOGICAL PROFILE AND ANTIBIOTIC SENSITIVITY PATTERN OF HOSPITALACQUIRED SEPTICEMIA IN A TERTIARY CARE HOSPITAL IN NORTH EAST INDIA. Asian Journal of Pharmaceutical and Clinical Research, 10(11), 186. https://doi.org/10.22159/ajpcr.2017.v10i11.20554
How to Cite
License
Copyright (c) 2023 The Evidence

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright© by the author(s). Published by the Evidence Journals. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Most read articles by the same author(s)
- Krati Agarwal, Shriyansh Srivastava, Vanya Singh, Ranjana Rohilla, Kamran Zaman, Atul Rukadikar, Parul Singh, Vivek Hada, Aroop Mohanty, Rama Shankar Rath, Surekha Kishore, Ranjit Sah, One health concept and its applications in clinical practice: a comprehensive review , The Evidence: Vol. 2 No. 1 (2024): JAN - MAR
- Pawan Kumar, Ahmad Neyazi, Roy Rillera Marzo, Celso Augusto Guimaraes, Joshuan J Barboza, Hashem Abu Serhan, Adnan Kisa, Sarath Lekamwasam, Vasso Apostolopoulos , Alfonso J. Rodríguez-Morales, Ranjit Sah, Mycoplasma pneumoniae returns: understanding its spread and growing impact , The Evidence: Vol. 2 No. 1 (2024): JAN - MAR
- Aroop Mohanty, Malathi Mini, Abdullah Zaawari, Aritra Banerjee, Rowanti Neha Bage, Tathagata Jha, From avian to human: understanding the cross-species transmission and the global spread of highly pathogenic avian influenza , The Evidence: Vol. 2 No. 2 (2024): APR - JUN
- Meenu Singh, Joseph L. Mathew, Ranjit Sah, Celso Augusto Guimarães Santos, Adnan Kisa, Alfonso J. Rodríguez-Morales, Ashish Joshi, Jagdish Khubchandani, Jugal Kishore, Roy Rillera Marzo, Atin Adhikari, Abdulai Abubakari, Aditya Singh, Ahmad Neyazi, Aroop Mohanty, Russell Kabir, Vasso Apostolopoulos, Abhishek Mewara, Aravind P Gandhi, Bijaya K Padhi, Navigating the future of academic publishing: mission and vision of "The Evidence" , The Evidence: Vol. 1 No. 1 (2023): OCT - DEC