approval [2,10]. Overall, these studies underscore the transformative potential of microneedle patches in enhancing drug delivery efficiency, improving patient comfort, and expanding healthcare accessibility.
Figure 1 highlights the potential applications of microneedle technology in healthcare, with a focus on targeted drug delivery systems, depicted through detailed anatomical and physiological illustrations including the skin's response to microneedle penetration and the related biological processes.
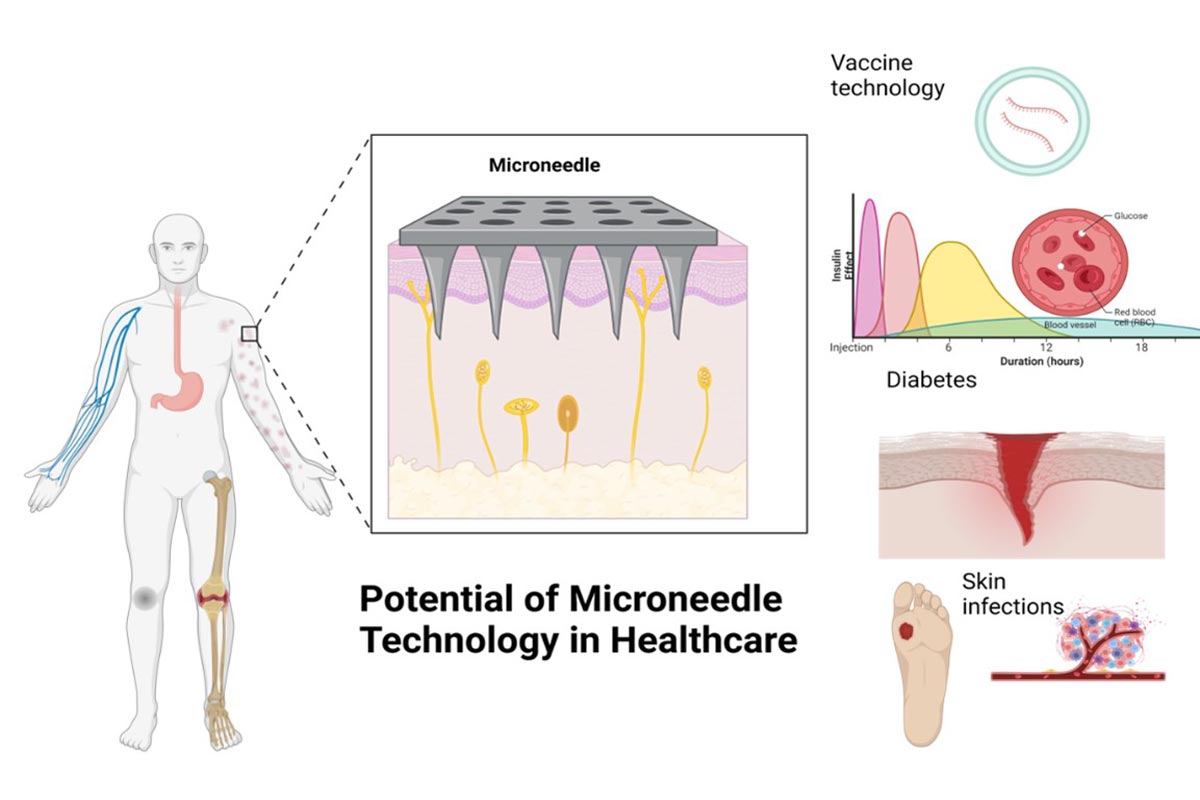
Figure 1: Potential applications of microneedle technology in healthcare
Revolutionizing Vaccine Delivery
The concept of microneedles was proposed in the 1970s, but it wasn't until the 1990s that it was experimentally demonstrated. This was made possible by the microfabrication tools provided by the microelectronics industry, which allowed for the creation of such small structures. Since the first studies on transdermal drug delivery in 1998 there has been a growing interest in the field [11]. Most of the activity has been focused on the development of novel needle fabrication technologies within the microfabrication community and the creation of microneedles for pharmaceutical applications within the drug delivery industry [12].
Transdermal drug delivery is a noninvasive method of administering biologically active agents through the skin for local or systemic effects. It can be self-administered and offers several advantages. However, there are certain requirements for drugs that are suitable for transdermal administration. For example, the maximum molecular weight should not exceed 1000 Da, and there should be a balance between hydrophobicity and polarity to overcome the barrier posed by the stratum corneum [13].
Most protein and peptide drugs, due to their hydrophilic and macromolecular nature, face difficulties in penetrating the skin. In recent decades, various chemical and physical methods have been developed to enhance transdermal drug permeation. These methods include the use of penetration enhancers [14] (Masterson 2020), microjet technology [15], laser [13], electroporation [16], sonophoresis [17], and iontophoresis [18]. However, these techniques are often expensive and cumbersome to use, and their efficiency in facilitating the successful transdermal delivery of macromolecular drugs remains limited [19].
One of the most promising applications of microneedle technology is in vaccine administration. Traditional vaccination methods often face challenges such as the need for skilled healthcare workers, the risk of needle-stick injuries, and the complexities of biomedical waste management.
